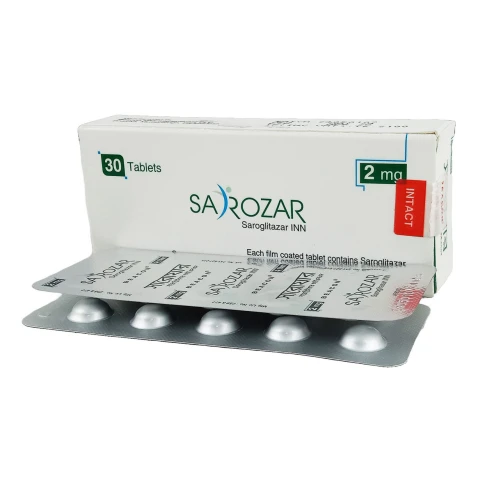
Sarozar 2 mg Tablet 30 pcs
৳ 2,0582,100
Quantity:
1
Brand: Beacon Pharmaceuticals Ltd.
SKU: BEA
Order By Call:
+88 01336 242 802 (Mobile)
+88 01336 242 802 (WhatsApp)
Indications
Sarozar tablet is indicated for the treatment of diabetic dyslipidemia and hypertriglyceridemia with Type 2 diabetes mellitus not controlled by statin therapy. In clinical studies, Sarozar tablet has demonstrated reduction of triglycerides (TG), Low Density Lipoprotein (LDL) cholesterol, Very Low Density Lipoprotein (VLDL) cholesterol, non-High Density Lipoprotein (non- HDL) cholesterol and an increase in HDL cholesterol. It has also shown favorable glycemic indices by reducing the fasting plasma glucose and glycosylated hemoglobin in diabetic patients.
About the Product
Full Description
Dosage & Administration
The recommended dose of Saroglitazar is two tablets of 2 mg once a day.
Pediatric Use: The safety and effectiveness of Saroglitazar in children less than 18 years of age have not been established.
Geriatric Use: Considering the comorbidity and concomitant medications in elderly patients, Saroglitazar should be used with caution in geriatric patients.
Pediatric Use: The safety and effectiveness of Saroglitazar in children less than 18 years of age have not been established.
Geriatric Use: Considering the comorbidity and concomitant medications in elderly patients, Saroglitazar should be used with caution in geriatric patients.
Side Effects
In two controlled phase III clinical studies of 12 to 24 weeks treatment duration with Sarozar, the most common adverse events (AEs > 2%) reported were gastritis, asthenia and pyrexia. Most of the AEs were mild to moderate in nature and did not result in discontinuation of the study. Because clinical studies are conducted under widely varying conditions, AE rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Reviews:
No Review
Releted Products